J. Feng & W. Q. Ma & H. H. Niu & X. M. Wu & Y. Wang & J. Feng
Abstract Three hundred sixty healthy Ross×Ross 1-day-old broilers were used to study the effects of zinc glycine chelate (Zn-Gly) on growth performance, hematological, and immunological characteristics. All broilers were randomly assigned into six treatments. Diets were as follows: (1) control (containing 29.3 mg Zn kg-1 basic diet [0–3 weeks] and 27.8 mg Zn kg-1 [4–6 weeks]); (2) basic diet plus 30 mg Zn kg-1 from Zn-Gly; (3) basic diet plus 60 mg Zn kg-1 from Zn-Gly; (4) basic diet plus 90 mg Zn kg-1 from Zn-Gly; (5) basic diet plus 120 mg Zn kg-1 from Zn-Gly; (6) positive control, basic diet plus 120 mg Zn kg-1 from zinc sulfate (ZnSO4). After the 21- and 42- day feeding trials, the results showed that both of Zn-Gly and ZnSO4 could improve the growth performance of broilers, with the greatest average daily feed intake observed in the broilers fed 90 mg Zn kg-1 from Zn-Gly, but the greatest average daily gain observed with 120 mg Zn kg-1 from Zn-Gly (0–3 weeks kg-1) and 90mg Zn kg-1 from Zn-Gly (4–6 weeks). Adding additional Zn-Gly improved the levels of immunoglobulins (IgA, IgM, and IgG) and the contents of total protein and Ca in serum and increased the immune organs index especially with 90 mg Zn kg-1 as Zn-Gly. However, there were no significant differences in responses to complements (C3 and C4) and albumin in serum among the treatments.
Keywords: Zn-Gly · ZnSO4 Growth Hematological Immunological Broiler
Introduction
Zinc (Zn) is a nutritionally essential trace element that functions in numerous metabolic pathways. It plays a critical role in immune function as moderate Zn deficiency and severe Zn deficiency are known to affect immunity in human and animal models [1–4]. The most commonly used Zn for supplementation in animal diet is inorganic Zn in the form of zinc sulfate due to cost and commercial availability. It is a common practice in the broiler industry to formulate diets to contain 100–120 mg supplemental Zn kg-1. Burrell et al. [5] reported improved performance when broilers consumed diets formulated to contain 110mg Zn kg-1; the NRC (1994) requirement of Zn for broiler chickens is only 40 mg/kg. Excessive supplement of inorganic Zn can bring on serious environment pollution resulting from the low utilization of this element. Therefore, excretion of Zn may be reduced by using sources of Zn with higher availability. Zinc from amino acid complexes has been reported to be more bioavailable than Zn from inorganic sources [6, 7]. Spears [8] reported that ruminants have a similar efficiency in absorbing Zn from zinc Methionine and ZnO, but more Zn from zinc Methionine are retained. In addition, Kidd et al. [9] implied that dietary Zn from organic sources might be absorbed intact and function differently than Zn from inorganic sources after absorption.
Zinc methionine has been shown to be more bioavailable than zinc sulfate and zinc oxide when fed to chicks in either purified crystalline amino acid, semi-purified (soy isolate), or complex corn–soybean diets. Glycine (Gly) has a slightly higher stability constant for Zn than methionine [10] and this could result in the Zn-Gly complex being more available for absorption. Our recent study found that the appropriate dosage of Fe-Gly could improve performance, hematological, and immunological characteristics in weanling pigs [11]. But few studies have been conducted on Zn-Gly in broilers. Therefore, the main objective of the study was to investigate the effects of Zn-Gly on growth performance, immune function, and hematological characteristics in broilers.
Materials and Methods
Animals and Treatments
A total of 360, 1-day-old Ross×Ross broiler chicks were assigned to 18 pens in a completely randomized design. There were three pen replicates of 20 chicks in each of six treatment combinations. The basal maize–soybeans meal diet (Table 1) was formulated to meet or exceed the NRC [12] requirements for starting chicks except for Zn. Dietary treatments included the basal supplemented with 30, 60, 90, or 120 mg Zn kg-1 as reagent grade zinc glycine and another with 120 mg Zn kg-1 from zinc sulfate.
Prior to the experiment, the broiler house was disinfected with whitewash and fumigated with formalin gas. Feeders and drinkers were properly disinfected with 5% KMnO 4 solution and dried under direct sunlight. Room temperature was maintained at 35±1°C during the first week and gradually decreased to 24°C by the end of the third week. A 24-h light regime was provided throughout the experimental period. In the 42-day study, each pen was offered the respective diet and all broilers were given ad libitum access to feed and water.
Data and Sample Collection
The experiment was terminated at the 21st and 42nd days; average daily gain (ADG), average daily feed intake (ADFI), and feed/gain ratio (F/G) was monitored as terms of broiler performance. On days 21 and 42, two birds from each replicate were randomly selected and slaughtered to record data on the weight of immune organs (spleen, thymus, and bursa of fabricius); serum samples were isolated from the blood by centrifuging at 3,000×g for 10 min and stored at -70°C until analysis.
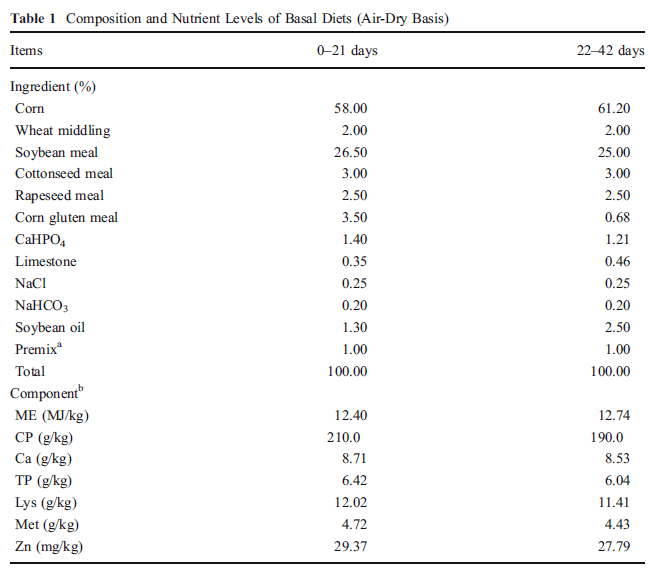
a Supplied the following per kilogram of diet: vitamin A, 25,000 IU; vitamin D, 5,000 IU; vitamin E, 12.5 IU; vitamin K, 2.5 IU; vitamin B1, 1.0 mg; vitamin B 2, 8.0 mg; vitamin B 6, 3.0 mg; vitamin B12, 15 µg; folic acid, 250 µg; nicotinic acid, 17.5 mg; calcium pantothenate, 12.5 mg; Fe, 80 mg; Cu, 10 mg; Mn, 80 mg; Se, 0.15 mg; I, 0.35 mg
b DE based on calculated values, others were analyzed values
Chemical Analyses
Concentrations of serum total protein, albumin, Ca, and P were assayed by an automatic biochemistry analyzer (RX-DAYTONA) using diagnostic kits manufactured by Sigma Diagnostic, Baroda, India. Immunoglobulins (IgA, IgG, and IgM) and complements (C3 and C4) concentrations were determined using an indirect enzyme-linked immunosorbent assay as described by Tiemann et al. [13]. Briefly, plates were incubated at room temperature for 60 min and then washed five times in washing solution. Then, 100 µL of peroxidase-conjugated goat antipig IgG (A100-104P) diluted 1:150,000, peroxidaseconjugated goat antipig IgM (A100-100P) diluted 1:40,000, or peroxidase-conjugated goat antipig IgA (A100-102P) diluted 1:100,000 in solution for samples was added to each well.
Plates were incubated at room temperature for 60 min and washed five times with washing solution. After incubation, unbound peroxidase conjugates were removed, and each well was washed five times with washing solution. Bound peroxidases were determined with TMB (0.1 mg/mL) and H2O2 (0.006%) in 0.1 M acetate, pH 6.0; and 100 µL of this substrate was transferred to each well. After 15 to 60 min incubation, the TMB reaction was stopped by addition of 100 µL of 2M H2SO4 to each well, and the optical density was measured at 450 nm with a microplate reader.
Statistical Analysis
Data were analyzed by analysis of variance as a randomized complete block design using the GLM procedures of SAS [14]. Pen was used as the experimental unit for growth performance data, and individual broilers were the experimental unit for other indices. The planned single degree of freedom tests included the linear and quadratic effects of Zn-Gly, the control versus ZnSO4 (120 mg/kg Zn), and ZnSO4 versus Zn-Gly treatments. A significant level of 0.05 was used for estimating differences among treatments.
Results
Growth Performance
Table 2 displays the effects of Zn-Gly supplementation on broiler performances. During 0-21 days, both Zn-Gly and ZnSO4 could improve the growth performance of broilers, and significant differences (P<0.05) were observed among treatments except ADG. The greatest final weight, ADFI, and F/G were observed in the broilers fed 120, 90, and 120 mg Zn kg-1 as Zn-Gly, respectively. However, there was no difference between Zn-Gly and ZnSO4 with same supplementation (P>0.05). Over the latter trial period (22–42 days), all the greatest final weight, ADG, ADFI, and F/G were observed in the broilers fed 90 mg Zn kg−1 (P<0.01) as Zn-Gly. In addition, the effect of adding 120 mg ZnSO4 was similar as adding 60 mg Zn-Gly (P>0.05).
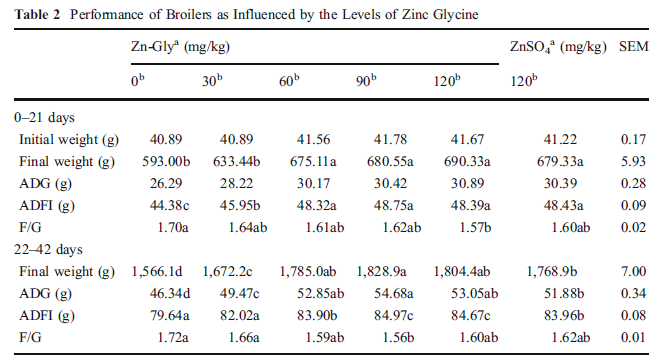
Within each period, means in a row not sharing a common letter differ (P<0.05) SEM standard error of mean, ADG average daily gain, ADFI average daily feed intake, F/G feed/gain ratio
a Zn source
b Zn addition (in milligrams per kilogram)
Hematological and Immunological Characteristics
The effects of different levels of Zn as Zn-Gly on immune organs index are presented in Table 3. During 0-21 days, thymus, spleen, and bursa of fabricius indexes increased linearly with increasing dietary Zn-Gly levels and reached a peak mainly in 90 mg/kg (P<0.01) Zn as Zn-Gly groups. However, no difference was observed between the 120 mg/kg Zn-Gly group and 120 mg/kg ZnSO4 group at days 21 and 42 (P>0.05). There were no significant differences on spleen and bursa of fabricius indexes to 6-week-old broilers among the treatments yet (P>0.05).
The different levels of Zn also affected serum immunoglobulins and complements concentrations (Table 4). Specifically, the levels of Zn-Gly increased the IgA concentration in serum over the whole trial period and significantly higher than the control when 90 mg Zn as Zn-Gly was added in the latter period (22-42 days; P<0.05). Moreover, 90 mg Zn-Gly also improved the levels of IgG and IgM concentrations especially during0–21 days (P<0.05), but no significant differences were noted for the complements (C3 and C4) over the whole period (P>0.05). Moreover, the effects of Zn-Gly and ZnSO4 in the serum immunoglobulins and complements concentrations were similar (P>0.05).
The effects of different levels of zinc glycine on total protein (TP), albumin (ALB), Ca, and P in broilers were shown in Table 5. Adding additional Zn (Zn-Gly orZnSO4) improved the levels of TP, Ca, and P especially with 90 mg Zn kg-1 as Zn-Gly in the latter period (22- 42 days) (P<0.01). In addition, Zn status also affects the contents of ALB in serum, whereas no significant differences were observed over the whole trial period (P>0.05).
Discussion
The present experiment examined broilers responses to different dietary levels of Zn-Gly. When diet Zn levels were >60 mg/kg, there were significant changes of body weight and ADFI, especially for 90 mg/kg which has the greatest effect on growth performance of broilers. Consistent with our results, a number of researchers documented that dietary zinc supplementation increased feed intake, growth rate, and feed efficiency in broiler chicks [15-18] and quail [19]. Reports show that diets with low zinc lead to depressed appetite, resulting in decreased feed intake and reduced weight gain [20-22]. Zinc is required for the biological function of more than 300 enzymes. In particular, Zn is essential and directly involved in catalysis and cocatalysis by the enzymes, which control many cell processes including DNA synthesis, normal growth, brain development, behavioral response, reproduction, fetal development, membrane stability, bone formation, and wound healing [23, 24]. Additionally, Zn has been suggested to be required by the fetus for support of cell proliferation and tissue differentiation of developing organs [25]. Supplementation with zinc improves performance of animals such as poultry, swine, and dairy animals as measured final body weight or feed efficiency. Although the NRC [12] recommends a minimum of 40 mg of zinc/kg of diet, clinical signs of zinc deficiency have been observed even when dietary zinc was increased above the recommended minimum levels [26]. In the current experiment, the basal diet contained 29.37 mg Zn kg-1 (0-21 days) and 27.79 mg Zn kg-1 (22-42 days), which could not meet the requirement for broilers. Low Zn concentration in basal diet may contribute partly to the increasing effect of Zn-Gly on broiler ADG. The present study also showed that the effect of a moderate dosage Fe from Zn-Gly (90 mg/kg) on growth performance was numerically better than that of 120 mg/kg Zn from ZnSO4. The apparent deficiency can be explained in part by reduced bioavailability resulting from dietary antagonists and interaction with other minerals. In production animals, organic zinc sources such as zinc methionine or Zn-Gly were more bioavailable than inorganic zinc sources such as ZnO or ZnSO4 [6, 8, 27], which needs further study.
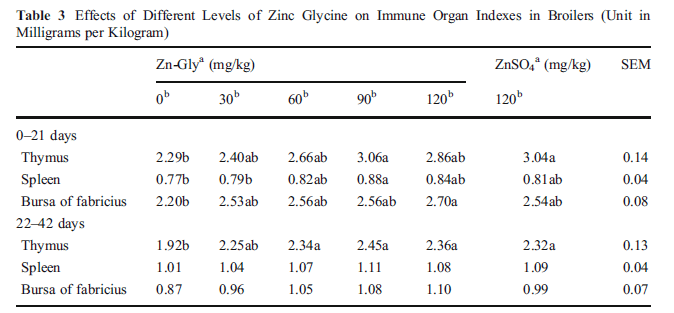
Within each period, means in a row not sharing a common letter differ (P<0.05)
SEM standard error of mean
a Zn source
b Zn addition (in milligrams per kilogram)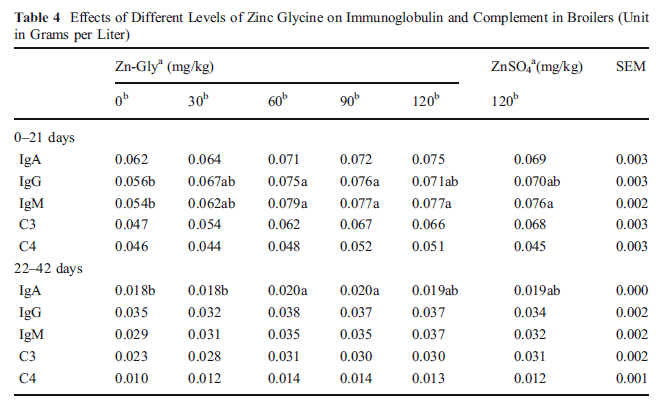
Within each period, means in a row not sharing a common letter differ (P<0.05)
SEM standard error of mean
a Zn source
b Zn addition (in milligrams per kilogram)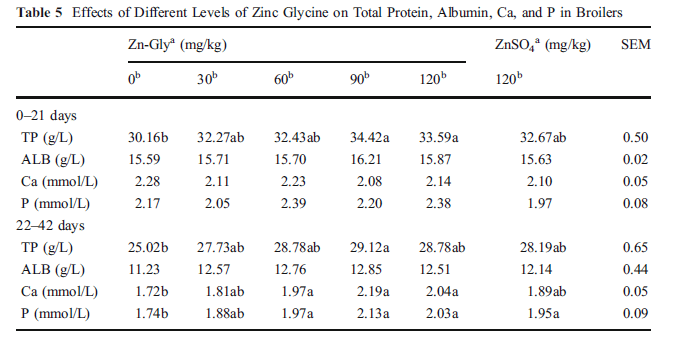
Within each period, means in a row not sharing a common letter differ (P<0.05)
SEM standard error of mean, TP total protein, ALB albumin
a Zn source
b Zn addition (in milligrams per kilogram)
Zinc, which interacts with the components of the immune system, is considered crucial for immune responses [28]. It bounds to enzymes, proteins, and peptides with different binding affinities [24]. Immune organ weights were measured as a percentage of body weight in this study. All these organs (thymus, spleen, and bursa of fabricius) were significantly affected by the level of Zn-Gly in the diet to 3-week-old broilers. This finding was consistent with previous reports that a diet deficient in zinc leads to atrophy of the thymus [29] and a reduction in spleen weight of rats [30]. Supplementing zinc in the diets did not seem to improve the weight of these organs in 6-week-old broilers except for the thymus. This could have been a result of more nutrients being repartitioned to develop body weight, and the immune system needs a relatively small amount of nutrients in relation to what is needed for growth [31]. Moreover, immunity of organs was not fundamental for the birds in the later growth phase (22-42 days).
In the present study, birds receiving the diets with high levels of Zn-Gly had significantly higher concentration of immunoglobulin and complements. These results were consistent with previous reports [32, 33] that diets supplemented with ample zinc tend to improve the ability of producing antibodies. Stahl et al. [34] also reported that zinc as Zn-Met supplementation (100 mg/kg Zn to a basal diet containing 36.8 mg Zn kg-1 DM) had better effect on the primary immune response to SRBC relative to controls. In contrast to the present study, Pimenteletal. [35] reported that broilers supplemented with 80 mg Zn kg-1 DM from ZnSO4 to a diet containing 34.7 mg Zn kg-1 DM had lower antibodytiter concentrations to SRBC than the control. However, in another study utilizing 7-day-old broilers, Wellinghausen et al. [36] reported that the total nonspecific IgM and IgG concentrations were affected by Zn status in zinc methionine and zinc sulfate. Fraker et al. [37] also reported that supplementing Zn as ZnSO4 to a diet deficient in Zn was effective on total serum IgG concentration. The reasons for these discrepancies between experiments are unclear. Because of the lack of sufficient evidence on the use of these zinc glycine complexes and their effect on the immune system, we can only postulate that the level of zinc could be manipulated further before a definite recommendation is made.
It was found that zinc glycine increased serum total protein and calcium concentration but had no effect on albumin or phosphorus. Calcium as an important regulator of lymphocyte function is involved in the signal transduction of lymphocytes and promotes the proliferation of lymphocytes [38]. This response was similar to the observations of Burrell et al. [5]. The increased calcium induced by Zn-Gly may be of some significance in the immune modulation of broilers and the mechanism needs further investigation.
Conclusion
In summary, data from these trials indicated that the supplementation with Zn-Gly can improve the growth performance and immunological capacity of broilers. Further studies may be warranted to investigate the mechanism of absorption and transport of Zn-Gly in the body.
References
1. Keen CL, Gershwin ME (1990) Zinc deficiency and immune function. Annu Rev Nutr 10:415–431
2. Shankar AH, Prasad AS (1998) Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 68:447S–463S
3. Ibs KH, Rink L (2003) Zinc-altered immune function. J Nutr 133:1452S–1460S
4. Donmez H, Donmez HH, Keskin E, Celik I (2002) Effects of zinc supplementation to ration on some hematological parameters in broiler chicks. Biol Trace Elem Res 87:125–131
5. Burrell AL, Dozier WA 3rd, Davis AJ, Compton MM, Freeman ME, Vendrell PF, Ward TL (2004) Responses of broilers to dietary zinc concentrations and sources in relation to environmental implications. Br Poult Sci 45:255–263
6. Wedekind KJ, Hortin AE, Baker DH (1992) Methodology for assessing zinc bioavailability: efficacy estimated for zinc-methionine, zinc sulfate, and zinc oxide. J Anim Sci 70:178–187
7. Cao J, Henry PR, Guo R, Holwerda RA, Toth JP, Littell RC, Miles RD, Ammerman CB (2000) Chemical characteristics and relative bioavailability of supplemental organic zinc sources for poultry and ruminants. J Anim Sci 78:2039–2054
8. Spears JW (1989) Zinc methionine for ruminants: Relative bioavailability of zinc in lambs and effects of growth and performance of growing heifers. J Anim Sci 67:835–843
9. Kidd MT, Ferket PR, Qureshi MA (1996) Zinc metabolism with special reference to its role in immunity. World’s Poult Sci J 52:309–324
10. Kratzer FH, Vohra P (1986) Chelates in nutrition. CRC, Boca Raton
11. Feng J, Ma WQ, Xu ZR (2007) Effects of iron glycine chelate on growth, haematological and immunological characteristics in weaning pigs. Anim Feed Sci Technol 134:261–272
12. NRC (1994) Nutrient requirements of poultry, 9th edn. National Academies, Washington
13. Tiemann U, Brussow KP, Jonas L, Pohland R, Schneider F, Danicke S (2006) Effects of diets with cereal grains contaminated by graded levels of two Fusarium toxins on selected immunological and histological measurements in the spleen of gilts. J Anim Sci 84:236–245
14. SAS Institute (1988) SAS/STAT user’s guide. SAS Institute, Cary
15. Sadoval M, Henry PR, Littell RC, Miles RD, Butcher GD, Ammerman CB (1999) Effect of dietary zinc source and method of oral administration on performance and tissue trace mineral concentration of broiler chicks. J Anim Sci 77:1788–1799
16. Stahl JL, Greger JL, Cook ME (1990) Breeding hen and progeny performance when hens are fed excessive dietary zinc. Poult Sci 69:259–263 Feng et al.
17. Roberson KD, Edwards HM (1994) Effects of 1,25-dihydroxycholecalciferol and phytase on zinc utilization in broiler chicks. Poult Sci 73:1312–1316
18. Sandoval M, Henry PR, Luo XG, Littell RC, Miles RD, Ammerman CB (1998) Performance and tissue zinc and metallothionein accumulation in chicks fed a high dietary level of zinc. Poult Sci 77:1354–1363
19. Sahin K, Kucuk O (2003) Zinc supplementation alleviates heat stress in laying Japanese quail. J Nutr 133:2808–2811
20. Rossi P, Rutz F, Anciuti MA, Rech JL, Zauk NHF (2007) Influence of graded levels of organic zinc on growth performance and carcass traits of broilers. J Appl Poult Res 16:219–225
21. Swinkels JW, Kornegay ET, Verstegen MW (1994) Biology of zinc and biological value of dietary organic zinc complexes and chelates. Nutr Res Rev 7:129–149
22. Park SY, Birkhold SG, Kubena LF, Nisbet DJ, Ricke SC (2004) Review on the role of dietary zinc in poultry nutrition, immunity, and reproduction. Biol Trace Elem Res 101:147–163
23. Dołęgowska B, Machoy Z, Chlubek D (2003) Changes in the content of zinc and fluoride during growth of the femur in chicken. Biol Trace Elem Res 91:67–76
24. Mocchegiani E, Muzzioli M, Giacconi R (2000) Zinc and immunoresistance to infection in aging: new biological tools. Trends Pharmacol Sci 21:205–208
25. Hostetler CE, Kincaid RL (2004) Gestational changes in concentrations of selenium and zinc in the porcine fetus and the effects of maternal intake of selenium. Biol Trace Elem Res 97:57–70
26. Van den Broek AHM, Thoday KL (1986) Skin disease in dogs associated with zinc deficiency: a report of five cases. J Small Anim Pract 27:313–317
27. Hahn JD, Baker DH (1993) Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J Anim Sci 71:3020–3024
28. Wellinghausen N, Rink L (1998) The significance of zinc for leukocyte biology. J Leukoc Biol 64:571–577
29. Yu ZP, Le GW, Shi YH (2005) Effect of zinc sulphate and zinc methionine on growth, plasma growth hormone concentration, growth hormone receptor and insulin-like growth factor-I gene expression in mice. Clin Exp Pharmacol Physiol 32:273–278
30. Mengheri E, Bises G, Gaetani S (1988) Differentiated cell-mediated immune response in zinc deficiency and in protein malnutrition. Nutr Res 8:801–812
31. Bartlett JR, Smith MO (2003) Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci 82:1580–1588
32. Beach RH, Gershwin ME, Hurley LS (1980) Impaired immunological ontogeny in postnatal zinc deprivation. J Nutr 110:805–810
33. Burns RM (1983) Antibody production suppressed in the domestic fowl (Gallus domestics) by zinc deficiency. Avian Pathol 12:141–146
34. Stahl JL, Cook ME, Sunde ML, Greger JL (1989) Enhanced humoral immunity in progeny chicks from hens fed practical diets supplemented with zinc. Appl Agric Res 4:86–89
35. Pimentel JL, Cook ME, Greger JL (1991) Immune response of chicks fed various levels of zinc. Poult Sci 70:947–954
36. Wellinghausen N, Kirchner H, Rink L (1997) The immunobiology of zinc. Immunol Today 18:519–521
37. Fraker PJ, King LE, Laakko T (2000) The dynamic link between the integrity of the immune system and zinc status. J Nutr 130(5S Suppl):1399S–1406S
38. Imboden JB, Weiss A, Stobo JD (1985) The antigen receptor on a human T cell line initiates activation by increasing cytoplasmic free calcium. J Immunol 134:663–665

